Imagine turning a series of blurry MRI slices into a crystal-clear 3D map of the liver in seconds. No more guesswork or manual tracing.
Liver segmentation MRI uses advanced algorithms to isolate liver tissue from surrounding organs, creating precise 3D “masks” for volumetry, radiomic analysis, and treatment planning. Whether measuring liver volume for surgery or extracting texture features for personalized diagnostics, segmentation transforms raw scans into actionable insights.
Discover classic methods, cutting-edge AI techniques, essential tools, and smart tips to master liver segmentation MRI from start to finish.

What Is Liver Segmentation MRI?
Liver segmentation MRI is the process of isolating the liver from surrounding tissues on magnetic resonance images. In practice, it means drawing or computing a precise boundary around the liver in each slice of an MRI scan, then stacking these outlines to form a 3D map of the organ.
The key goal of liver segmentation MRI is to generate accurate three-dimensional liver masks. These masks serve as the foundation for quantitative analyses, such as measuring total liver volume for surgical planning and for extracting radiomic features that guide personalized treatment decisions.
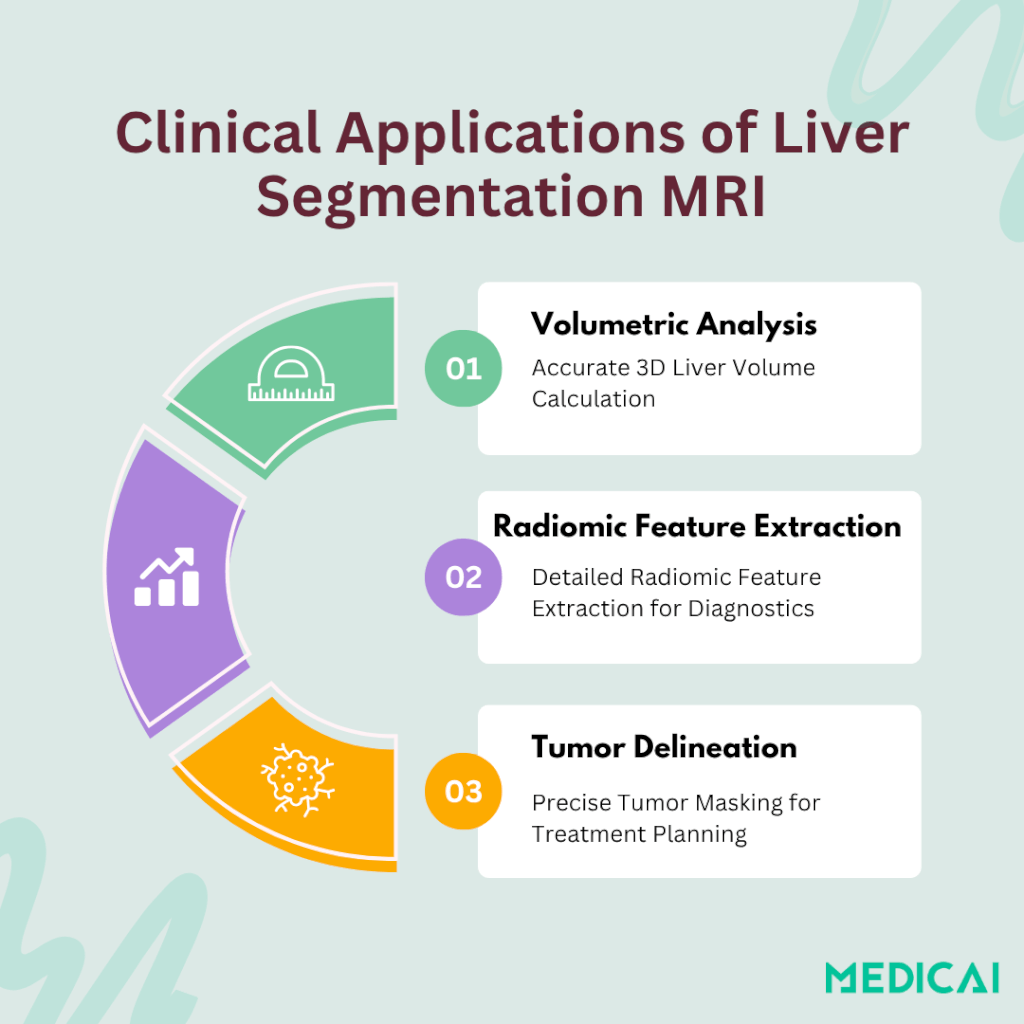
Why Liver Segmentation MRI Matters in Modern Radiology
Accurate liver segmentation MRI underpins several critical radiology workflows, transforming raw scan data into actionable insights.
Volumetric Analysis for Surgical Planning & Transplant Assessment
By converting segmented slices into a 3D liver mask, clinicians can calculate total liver volume with high precision. This volumetry guides decisions for resection margins in tumor surgery and helps assess donor–recipient size match in living-donor liver transplants.
Radiomic Feature Extraction for Personalized Diagnostics
Once a liver mask is established, hundreds of quantitative features, such as texture, shape, and intensity distributions, can be extracted. These radiomic signatures correlate with fibrosis stage, steatosis, and treatment response, enabling tailored patient management plans.
Tumor Delineation in HCC and Metastases
Segmentation MRI also isolates lesions from healthy parenchyma, improving detection and boundary definition of hepatocellular carcinoma (HCC) and metastatic disease. Precise tumor masks support accurate dose planning in radioembolization and targeted therapies, reducing collateral damage to normal tissue.
MRI Acquisition & Preprocessing for Liver Segmentation
Preparing MRI scans properly helps in accurate liver segmentation. This involves selecting optimal contrast phases and thorough image preprocessing before analysis.
Contrast Phases & Agents
Portal-venous T1-weighted MRI highlights liver parenchyma shortly after contrast injection, creating clear organ boundaries for segmentation algorithms. Hepatobiliary imaging with hepatocyte-specific agents like gadoxetate disodium further enhances healthy tissue contrast. The process improves delineation between liver, lesions, and vessels.
Image Preprocessing Workflow
Voxel resampling standardizes slice thickness and in-plane resolution, guaranteeing that each pixel represents consistent real-world dimensions.
Intensity normalization helps fix differences in brightness that can happen when using different scanners or between different patients. This process allows models to focus on the actual features of tissues instead of technical issues.
Finally, motion correction and fat–water swap fixes address breathing artifacts and misclassifications, sharpening liver boundaries on MRI.
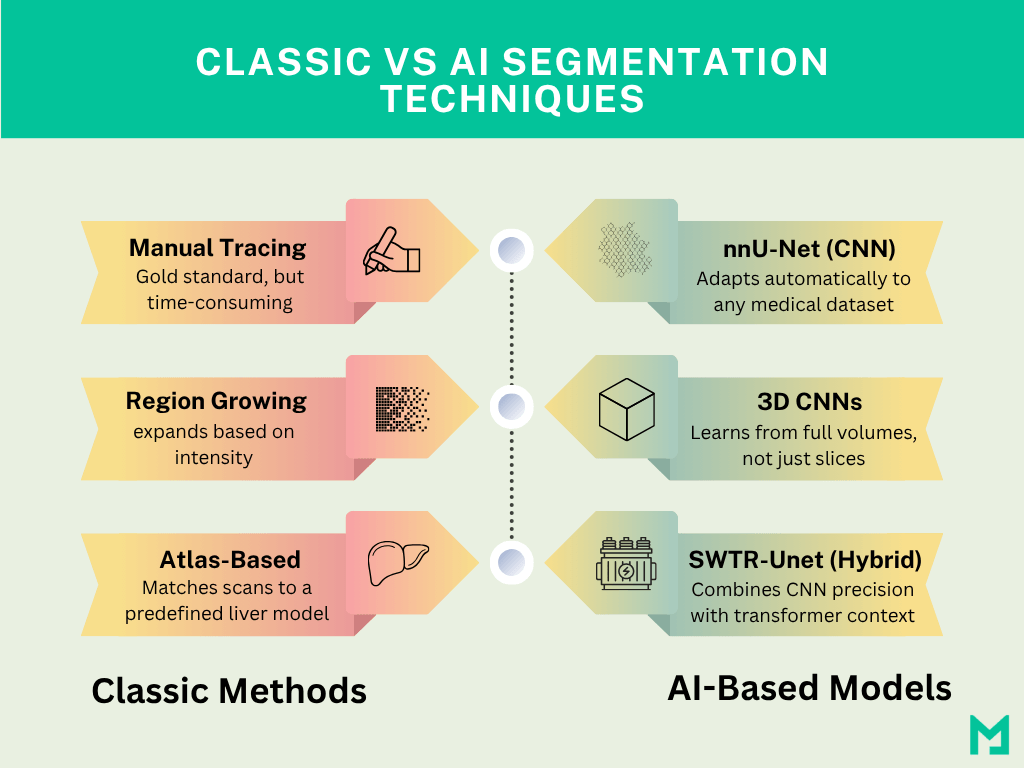
Traditional Methods for Liver Segmentation on MRI
Early liver segmentation on MRI relied on hands-on techniques and basic algorithms before the rise of deep learning approaches.
Manual & Semi-Automated Liver Segmentation
Manual tracing and assisted tools help outline the liver on MRI, but each has trade-offs in speed and effort.
- Hand-drawing (Gold Standard): Radiologists draw the liver edge on every image slice by hand. This delivers very precise boundaries but can take up to an hour per scan, and results vary between users.
- Seeded Region-Growing: Think of dropping two seeds—one inside the liver, one outside. The algorithm “grows” outward from each seed, filling similar intensities until the liver border emerges. It’s faster than manual work, but sometimes leaks into nearby organs.
- Graph-Cut & Deformable Models: Graph-cut treats segmentation like a puzzle, finding the smoothest boundary that best matches the data. Deformable models start with a rough shape that “snaps” onto the liver edges as it iterates. Both speed things up (“smart fill”) but may need extra tweaking if edges are blurry.
Shape- and Atlas-Based MRI Segmentation
Template-driven methods use pre-built liver maps to guide MRI segmentation, reducing manual effort but risking mistakes in unusual anatomy.
Statistical Shape Models: These learn the typical liver outline from many examples. When applied to a new scan, the model deforms to fit the patient’s liver, like bending a cookie cutter to match a misshapen dough.
Atlas-Based Registration: A detailed, labeled liver volume (the “atlas”) is warped to match each patient’s MRI geometry. It works smoothly when livers look normal, but can misalign if tumors or deformities are large.
Both methods cut down on hand-drawing but may require manual correction when anatomy deviates from the templates.

Deep Learning Techniques for Liver Segmentation MRI
Deep learning brings powerful AI for MRI that learns liver shapes from many scans, automating and improving segmentation accuracy.
CNN-Based Models: nnU-Net & 3D Deep CNNs
Convolutional neural networks (CNNs) use layers of “filters” to spot liver tissue patterns in MRI images.
nnU-Net is a ready-to-use framework that adapts itself to any medical dataset. When trained on hundreds of T1-weighted liver MRIs, it consistently achieves Dice scores above 0.90, meaning over 90% overlap between its mask and the true liver outline.
3D Deep CNNs examine the scan as a full volume instead of separate slices. Research has achieved a Dice score of 0.97 on internal tests and 0.96 on external datasets, demonstrating very close agreement with expert tracings.
These CNNs learn directly from examples: the more labeled scans you provide, the better their liver masks become.
Hybrid & Transformer Models: SWTR-Unet
Hybrid models combine CNNs with transformer blocks, which help the AI see both local details and the big picture.
SWTR-Unet starts with a CNN backbone (ResNet) to capture textures, then adds transformer layers that link distant parts of the image. This design achieved average Dice scores of 0.98 for the liver and 0.81 for lesions, matching human experts on MRI data.
By blending methods, hybrid networks improve boundary precision, especially around tumors, making them ideal for clinical scenarios where every millimeter counts.
Platforms like Medicai streamline workflows and empower clinicians with rapid, reliable insights in automating MRI liver segmentation. Our AI-Copilot automates accurate preprocessing, produces high-accuracy 3D masks, extracts radiomic features, and integrates with PACS.
Common Challenges in MRI Liver Segmentation
Segmentation accuracy can suffer when MRI scans have artifacts or uneven signal. Simple protocols and smart fixes improve results.
Motion Artifacts & Blurring
Breathing during scans causes the liver to shift, blurring its boundaries. Using breath-hold imaging or respiratory gating techniques freezes motion. Post-scan, correction algorithms realign the blurred slices, sharpening the organ edges.
Partial-Volume Effects
When the slice thickness is too large, a single voxel may contain liver and non-liver tissue, confusing segmentation algorithms. Acquiring thinner slices reduces this mixed-tissue problem, ensuring each voxel represents primarily one tissue type.
Intensity Inhomogeneity
MRI scanners can produce uneven brightness across images, making liver tissue appear variable. Advanced normalization methods—like bias field correction—flatten intensity gradients, so algorithms focus on true tissue differences rather than scanner quirks.
Conclusion
Liver segmentation MRI now combines smart image prep with both classic and AI methods. It helps clinicians quickly measure liver volume, extract radiomic data, and outline tumors with high precision.
Medicai helps with automating preprocessing and segmentation directly within your PACS. It delivers consistent, high-accuracy 3D liver masks and radiomic reports, freeing clinicians to focus on interpretation and patient care.




