What if we could see not just the heart’s structure, but how well it truly works? That’s exactly what nuclear cardiology makes possible.
Nuclear cardiology uses radioactive tracers to assess heart function, blood flow, and tissue viability. It enables early, noninvasive diagnosis of conditions like coronary artery disease and cardiac amyloidosis, offering more precise and personalized cardiac care.
Discover how technologies like PET, SPECT, AI, and novel radiotracers are reshaping the future of cardiac diagnostics

What Is Nuclear Cardiology?
Nuclear cardiology is a subspecialty of cardiovascular imaging that reveals how the heart functions. It uses trace amounts of radioactive substances (radiotracers) to track blood flow, measure heart muscle activity, and assess cardiac health from the inside out.
Unlike CT or MRI scans, which show the heart’s physical structure, nuclear cardiology focuses on metabolism, perfusion, and function. That means it can detect whether a part of the heart is getting enough blood, and whether it’s still viable, long before structural damage appears on a standard scan.
This approach has become essential in diagnosing complex or early-stage heart conditions. For example, a coronary artery may appear narrowed on an angiogram.
However, is that blockage truly restricting blood flow?
Nuclear cardiology answers that question with clarity.
It also allows for safer, noninvasive testing in patients who might otherwise need more invasive procedures, such as biopsies or catheterization.
Core Applications of Nuclear Cardiology in Clinical Practice
Nuclear cardiology is widely used to:
- Assess myocardial perfusion (blood flow to the heart muscle)
- Diagnose or monitor coronary artery disease (CAD)
- Evaluate damage after a heart attack
- Determine whether patients need stents or bypass surgery
- Monitor the effects of cardiotoxic treatments (like chemotherapy)
- Detect rare diseases like cardiac amyloidosis without requiring biopsy
Nuclear cardiology lets clinicians detect heart problems before symptoms become severe or noticeable. That’s why it is key in screening high-risk patients, tracking recovery after surgery, and optimizing long-term treatment strategies.
In essence, nuclear cardiology shifts the focus from how the heart looks to how the heart performs, and that shift saves lives.
From Biopsy to Breakthrough: Nuclear Imaging in Cardiac Diagnosis
Certain heart conditions, particularly cardiac amyloidosis, used to be diagnosed using a procedure called endomyocardial biopsy. While effective, it’s invasive, requires catheter insertion into the heart, and carries risks like bleeding, infection, or even perforation.
It also demands expert training and highly specialized centers.
Today, nuclear cardiology is changing that. Using targeted radiotracers, clinicians can detect amyloid deposits in the heart noninvasively, often with equal or greater accuracy, and long before symptoms become severe.
One of the most impactful shifts is diagnosing cardiac amyloidosis, a condition where misfolded proteins accumulate in the heart, making it stiff and less efficient. There are two main types:
- ATTR amyloidosis, linked to the transthyretin protein and often age-related
- AL amyloidosis, involving light chains from plasma cells
These subtypes require entirely different treatments, which makes early, accurate differentiation crucial. Nuclear imaging makes that possible.
Instead of tissue sampling, clinicians use bone-seeking tracers like 99mTc-PYP or 99mTc-DPD to diagnose ATTR with high confidence. When AL amyloidosis needs to be ruled out, lab tests for serum and urine proteins are added.
For more complex cases, PET tracers such as 11C-PiB or 18F-florbetaben offer even greater specificity, particularly in detecting AL involvement and monitoring treatment response.
What used to be a complex hospital-based biopsy can now be replaced by a scan and a few blood tests. This breakthrough is improving diagnostic timelines, patient comfort, and access to care.
More studies show that imaging tools diagnose and predict prognosis and track treatment effectiveness, enabling more personalized cardiac care.

Myocardial Perfusion Imaging (MPI): The Heart of Nuclear Cardiology
Myocardial perfusion imaging, or MPI, is one of the most established and trusted techniques in nuclear cardiology. It’s a noninvasive scan that evaluates how well blood flows to the heart muscle.
MPI compares perfusion at rest and during stress to help clinicians identify areas of the heart that may lack sufficient oxygen-rich blood before symptoms like chest pain or fatigue worsen.
This dual-phase approach is vital. When a heart region shows reduced blood flow under stress but looks normal at rest, it typically signals reversible ischemia. It’s a warning sign of potentially significant coronary artery disease (CAD).
On the other hand, if a defect is present in both stress and rest scans, it suggests scar tissue or previous myocardial damage.
The Science Behind the Scan
Radiotracers are central to MPI’s operation. Once injected into the bloodstream, these substances mimic blood flow patterns and accumulate in healthy heart tissue. Areas that take up less tracer appear as “cold spots” on the scan, indicating poor perfusion.
Common tracers used for MPI include:
- Technetium-99m compounds, such as sestamibi (MIBI) and tetrofosmin, are widely used in SPECT imaging due to good image quality and favorable half-life
- Thallium-201, an older tracer with a longer half-life, is still used in select cases for viability assessment
- PET tracers such as Rubidium-82, Nitrogen-13 ammonia, and the newly FDA-approved F18-Flurpiridaz offer high resolution for evaluating suspected CAD.
Each tracer has its strengths, but they all aim to do the same thing: show how well blood reaches the heart under different conditions.
Why MPI Matters in Clinical Practice?
MPI is more than just a diagnostic tool; it’s often the first scan to identify serious health issues. Clinicians rely on MPI to:
- Detect coronary artery disease (CAD), even before it causes noticeable symptoms
- Assess the severity of blood flow limitation, especially when physical or pharmacological stress unmasks hidden ischemia
- Evaluate the risk of future heart attacks and cardiac death
- Guide treatment strategies, such as choosing between medication, angioplasty, or bypass surgery
- Monitor recovery after a heart attack or surgical procedure
MPI holds significant prognostic value, with normal results indicating a low annual risk of cardiac events similar to the general population. When abnormalities are detected, the degree of reduced blood flow aids in risk stratification and influences treatment planning.
SPECT vs PET: Choosing the Right Modality
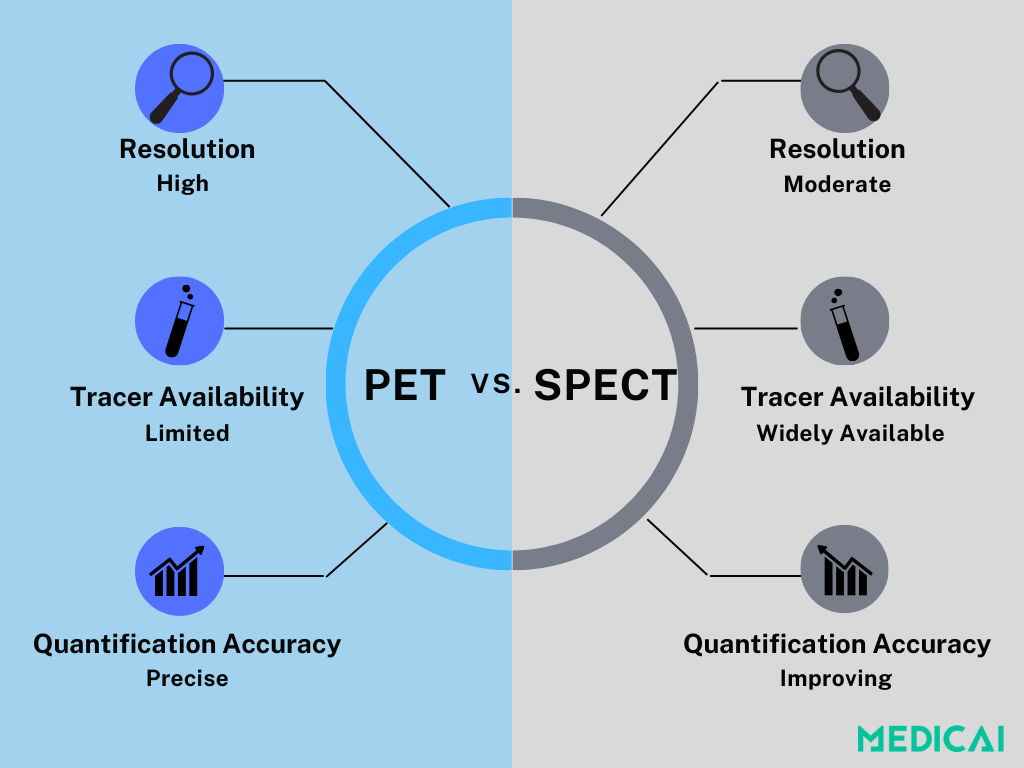
While both SPECT and PET are used in myocardial perfusion imaging (MPI), they differ in key areas: resolution, quantification accuracy, availability, and cost.
PET is the gold standard for quantifying myocardial blood flow (MBF). Its higher spatial resolution and ability to measure blood flow in absolute terms make it especially useful for evaluating complex or subtle coronary disease.
However, PET remains limited by cost and accessibility. Specialized tracers like Rubidium-82, N-13 ammonia, and F18-Flurpiridaz are expensive and unavailable. Many PET scanners are also prioritized for oncology, making cardiac use less feasible in many regions.
On the other hand, SPECT is far more accessible and uses widely available tracers like Technetium-99m MIBI and Tetrofosmin. Although it doesn’t match PET’s precision, newer technologies like CZT (Cadmium Zinc Telluride) detectors have significantly improved image quality, speed, and sensitivity.
With dynamic SPECT and AI-assisted reconstruction now under development, SPECT is gaining ground as a more practical, cost-effective option for MBF evaluation, particularly where PET access is limited.
As SPECT and CZT technologies advance, solutions like Medicai can improve image quality and standardize protocols, helping close the PET performance gap.
In the end, choosing between the two is a matter of balancing performance with practicality. PET delivers unmatched quantification, but SPECT is evolving fast, and for many clinics, it’s the most realistic path forward in nuclear cardiology.
Cardiac Amyloidosis: How Nuclear Imaging Is Redefining Diagnosis
Cardiac amyloidosis was traditionally diagnosed through biopsy, a risky and specialized procedure. Now, nuclear cardiology allows for noninvasive, earlier detection and classification of this complex condition.
Amyloidosis is caused by the buildup of misfolded proteins in the heart, which interfere with its ability to function properly. Two main types affect the heart:
- ATTR (transthyretin amyloidosis) – often age-related or hereditary
- AL (light chain amyloidosis) is associated with plasma cell disorders
These two types differ in pathology and treatment, so distinguishing between them is critical. Biopsy can do that, but it’s invasive. Nuclear imaging now offers a safer alternative.
The Rise of Bone-Avid Tracers
Using bone-seeking tracers like 99mTc-PYP, 99mTc-DPD, and 99mTc-HMDP has dramatically improved the diagnosis of ATTR amyloidosis. Strong radiotracer uptake in the heart (Grade 2 or 3) combined with regular blood tests for monoclonal proteins confirms the diagnosis without needing a biopsy.
This approach enhances accessibility and enables earlier disease detection, often before irreversible heart damage occurs.
PET Imaging: A New Frontier for AL Amyloidosis
While bone tracers are excellent for ATTR, they are less reliable for AL amyloidosis. This is where PET imaging comes into play.
Using tracers such as 11C-PiB, 18F-florbetaben, or 18F-florbetapir, PET can bind directly to amyloid proteins and visualize both AL and ATTR subtypes, with a tendency to show even greater uptake in AL cases.
Studies show PET helps differentiate amyloid types and provides insights into disease burden and treatment response. For AL amyloidosis patients on chemotherapy, changes in PET signal may indicate therapy effectiveness, revolutionizing disease monitoring.
Beyond Perfusion: The Expanding Toolkit of Nuclear Cardiology
Myocardial perfusion imaging (MPI) is central to nuclear cardiology. However, the field has expanded to include techniques for assessing myocardial metabolism, nerve activity, and tissue viability, enhancing cardiac diagnostics in heart failure care.
FDG-PET is one such tool. Imaging glucose uptake in the heart muscle helps detect hibernating myocardium. This tissue is alive but underperforming due to chronic low blood flow. The area may recover with revascularization if glucose is taken up despite weak contraction.
This makes FDG-PET especially valuable in patients with ischemic left ventricular dysfunction, guiding decisions about bypass surgery or other interventions.
Another emerging option is iodine-123 mIBG imaging, which evaluates the heart’s sympathetic innervation. Reduced mIBG uptake in heart failure patients has been linked to worse outcomes, offering clinicians an added tool for risk assessment and treatment planning.
Although not widely adopted, these techniques signify a shift from merely observing the heart’s structure to understanding its metabolic, electrical, and functional behavior. As more evidence emerges, they are set to enhance personalized cardiac care.
AI and Quantitative Imaging: The Next Leap Forward
As nuclear imaging evolves, AI simplifies and enhances the process. Scans provide detailed data on blood flow, function, and risk, making efficient data management crucial for quicker decision-making.
AI is particularly useful in quantifying myocardial blood flow (MBF), especially in PET imaging. It automates image reconstruction, segmentation, and flow analysis, reducing manual steps and variability between clinicians.
AI tools are streamlining image reconstruction, segmentation, and blood flow quantification. Platforms like Medicai integrate multi-modality data into real-time decision-support tools, reducing variability and enhancing diagnostic confidence.
AI also supports dynamic SPECT, where fast imaging is needed to estimate MBF. With better algorithms and detectors like CZT, SPECT is inching closer to PET-level capabilities.
Beyond quantification, AI is trained to predict outcomes by analyzing patterns across perfusion, function, and extracardiac uptake. These models could soon help clinicians stratify risk with greater accuracy.
AI isn’t replacing radiologists; it’s enhancing their work, making imaging smarter and faster, and leading to better personalized cardiac diagnostics.
Conclusion
Nuclear cardiology is transforming heart care by prioritizing function over anatomy, enabling earlier and more accurate diagnoses. Key advancements include perfusion imaging, AI-driven quantification, and amyloidosis detection through technologies like PET, CZT-SPECT, and functional imaging.
Platforms like Medicai help clinicians analyze cardiac data more accurately and efficiently. By blending automation with clinical insight, we enable personalized and scalable cardiac diagnostics.




