In a world where data moves fast, security in healthcare must move faster, protecting every medical image, every time, everywhere.
To share medical images for second opinions, healthcare providers must adhere to HIPAA and GDPR rules. It includes encrypting files, controlling access, maintaining audit trails, and allowing patients to securely share their data. These measures provide privacy while promoting swift collaboration among specialists globally.
Find out how to share medical images securely, stay compliant with global standards, and share images safely.

Regulatory Landscape: HIPAA & GDPR Primer
In healthcare, privacy isn’t optional; it’s the foundation of patient trust.
Every medical image, like an MRI or CT scan, contains personal data. That’s why HIPAA in the U.S. and GDPR in Europe establish strict standards for the secure handling, storage, and sharing of medical information.
HIPAA Basics: Protecting Patient Health Information in the U.S.
In North America, the Health Insurance Portability and Accountability Act (HIPAA) governs the protection of patient data, including medical images. It mandates that healthcare providers, insurers, and their associates safeguard all Protected Health Information (PHI) in both paper and electronic formats.
Digital sharing of medical images like X-rays, MRIs, or CT scans involves electronic Protected Health Information (ePHI), which often includes personal identifiers. HIPAA requires safeguards to protect this data.
It means implementing:
- Encryption for data both at rest and in transit.
- Access controls that restrict viewing privileges to authorized individuals.
- Audit trails to record who accessed the images, when, and what actions they took.
HIPAA violations can lead to fines ranging from thousands to millions based on severity and intent. Compliance is crucial to keep patient data secure, especially when a radiologist shares images for a second opinion.
DPR Basics: Safeguarding Personal Data in the EU and EEA
The General Data Protection Regulation (GDPR) applies to any organization processing personal data of EU or EEA citizens. These include hospitals, research institutions, and telehealth platforms outside the EU serving European patients.
Under GDPR, health data is classified as a “special category of personal data,” meaning it requires the highest level of protection. Organizations must have a clear lawful basis for processing such data, usually patient consent, vital interest, or legitimate medical necessity.
GDPR also introduces key data subject rights, such as:
- The right to access: patients can view the data stored about them.
- The right to rectification: they can request corrections to inaccurate information.
- The right to erasure (“right to be forgotten”): they can request deletion in certain cases.
- The right to data portability: they can obtain their data in a structured, machine-readable format and transfer it elsewhere.
Data portability in healthcare is a game-changer.
Data Portability: Empowering Patients to Share Medical Images Freely
Under Article 20 of the GDPR, patients have the right to receive their personal data and share it with providers of their choice without obstruction. It includes medical images and reports stored in DICOM or similar formats.
A patient in Germany can request their MRI scan and securely send it to a cardiologist in France or the U.S., as long as it meets security standards like encryption.
This concept empowers patients to share and take control of their healthcare by deciding who can access their data and for what purpose. Healthcare organizations must implement interoperable systems to securely share imaging data, avoiding breaches and compliance issues.
HIPAA vs. GDPR: Similar Goals, Different Scopes
While HIPAA and GDPR share a common goal, protecting sensitive health data, their scopes and applications differ.
| Aspect | HIPAA (U.S.) | GDPR (EU/EEA) |
| Who It Covers | Healthcare providers, insurers, and business associates | Any organization processing personal data of EU citizens |
| Type of Data | Protected Health Information (PHI/ePHI) | Personal data, including health data (special category) |
| Legal Basis | Authorization, treatment, or operations | Consent, legitimate interest, vital interest, etc. |
| Patient Rights | Limited (access, amendment, accounting) | Broad (access, erasure, portability, restriction) |
| Penalties | Up to $1.9 million per violation category per year | Up to €20 million or 4% of annual global turnover |
| Cross-Border Rules | U.S.-based, applies domestically | Applies extraterritorially, even outside the EU, if data subjects are EU citizens |
Both frameworks prioritize security by design with encryption, access control, and auditability. However, GDPR further emphasizes individual rights and data portability, empowering patients with greater control over their information.
Key Privacy & Security Challenges in Medical Image Sharing
Let’s explore the most pressing privacy and security challenges that healthcare organizations and patients face when sharing medical images.
Embedded Identifiers and Metadata Risks
Every DICOM image contains critical metadata like patient name, date of birth, hospital ID, scan date, and device information. If not anonymized, this data can expose patient identities during transfers.
“Burned-in” text, such as names or medical record numbers on images, also poses confidentiality risks.
Legacy Systems and Interoperability Gaps
Many hospitals use outdated PACS that lack external sharing and cloud access, with weak security protocols. This interoperability problem wastes radiologists’ time as they transfer data between incompatible systems.
Staff sometimes resort to unsafe methods, like burning images onto CDs or using unsecured file-sharing services.
The result? Convenience triumphs, but compliance suffers.
Unauthorized Access and Data Breaches
Healthcare data is a target for cybercriminals. Weak passwords and phishing can lead to breaches, allowing attackers to steal personal data or deploy ransomware, particularly in medical imaging systems.
Lack of Encryption During Transfers
When medical images travel between providers, from a hospital in Boston to a specialist in Toronto, data often passes through multiple networks. Without encryption in transit, this data can be intercepted or tampered with.
Encryption at rest keeps stored images unreadable without decryption keys. Both HIPAA and GDPR require sensitive health data, like medical images, to be protected and never stored in plain form.
Insufficient Audit Trails and Traceability
Audit trails are essential for compliance, showing who accessed files and their actions. Many healthcare systems fall short in logging, which complicates the tracking of unauthorized access and exposes organizations to legal risks while undermining patient trust.
Cross-Border Transfers and Jurisdictional Conflicts
Patients often seek second opinions from specialists in other countries—but moving medical data across borders introduces new complexities.
Under GDPR, data transfers outside the EU require additional safeguards, such as:
- Adequacy decisions (e.g., for countries with equivalent protections)
- Standard Contractual Clauses (SCCs) between organizations
- Explicit patient consent for the transfer
Meanwhile, HIPAA doesn’t prohibit international transfers but requires that Business Associate Agreements (BAAs) extend liability to any partner handling ePHI.
Without these measures, organizations risk breaching both laws, even if the transfer had a medical purpose.
Human Error and Unsecured Workarounds
Despite strict regulations, human mistakes remain the weakest link. Common examples include:
- Sending medical images to the wrong recipient
- Forgetting to remove personal identifiers
- Using unsecured USB drives or public cloud storage
- Sharing images over non-encrypted email chains
Errors can occur under time pressure or with difficult sharing systems. The solution isn’t just compliance; it’s about creating workflows that prioritize secure sharing.
Balancing Security with Accessibility
A key challenge is maintaining security while ensuring prompt care. Physicians need quick remote access to images for urgent consultations, and patients desire the ability to share scans freely.
If security protocols are overly complex, users may bypass them, increasing risk. The best systems offer seamless access while upholding strong security.

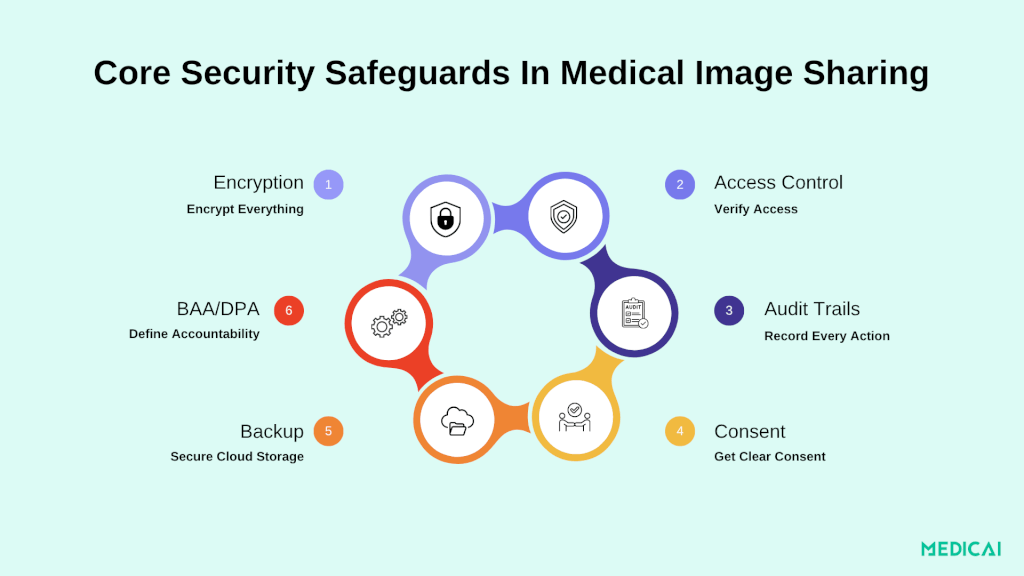
Essential Safeguards: Share Medical Images Securely
Several core technical and procedural measures make medical image sharing safe, HIPAA- and GDPR-compliant, and efficient for modern healthcare workflows.
Encryption: Protecting Data Everywhere
Encryption prevents unauthorized viewing of medical images.
- In transit: Secure data transfers with TLS 1.2+ to block interception.
- At rest: Use AES-256-bit encryption for stored data.
- End-to-end encryption (E2EE): Encrypt data on the sender’s side and decrypt only by the recipient—no middle access allowed.
Medicai uses rotating encryption keys and E2EE to safeguard data from upload to viewing.
Access Controls & Authentication
Only authorized users should see medical images.
- Role-Based Access (RBAC): Assign permissions by role (radiologist, patient, consultant).
- Multi-Factor Authentication (MFA): Add biometric or code verification.
- Time-Limited Access: Provide expiring or single-use access links.
Audit Trails & Logging
Accountability is vital for compliance.
Maintain immutable logs showing who viewed, downloaded, or shared an image. Generate automated alerts for suspicious activity.
Medicai offers tamper-proof audit logs, ensuring transparency during audits or investigations.
Data Minimization & De-Identification
Reduce what you share, keep only what’s necessary.
- Strip metadata like names or IDs from DICOM headers.
- Blur or crop identifiers in visible areas.
- Automate de-identification before sending images externally.
These steps preserve patient privacy without losing diagnostic accuracy.
Consent & Authorization
Patients must know who is accessing their data and why.
- Use digital consent forms for authorization.
- Allow patients to revoke access anytime.
Medicai’s workflows align with HIPAA’s authorization and GDPR’s explicit consent rules.
BAAs & DPAs: Defining Accountability
When third-party vendors handle data, clear agreements are required.
BAAs (HIPAA) and DPAs (GDPR) define roles, security duties, and breach responsibilities.
Medicai signs both, ensuring compliance across all partnerships.
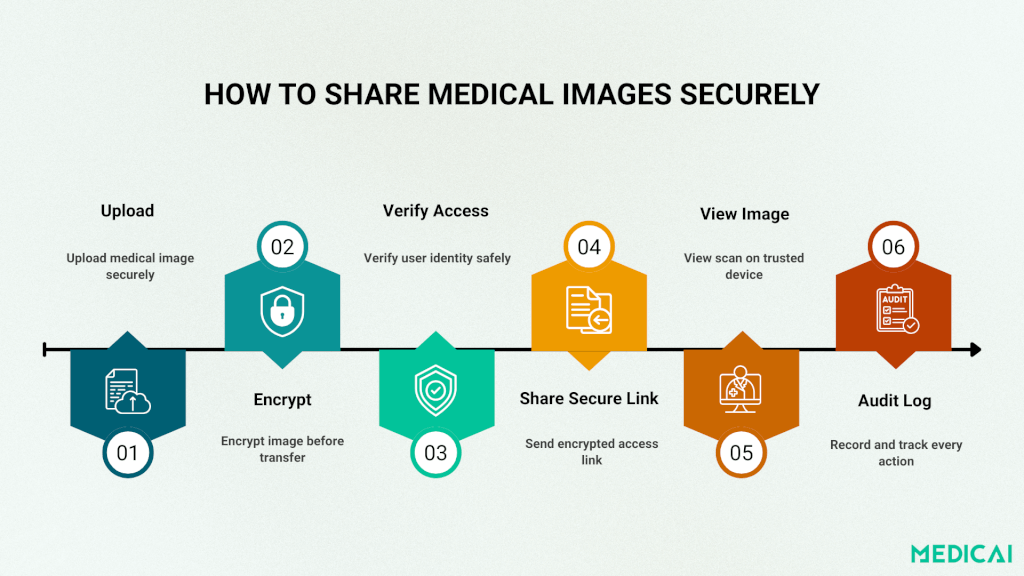
Zero Trust & Secure Transfers
Adopt a Zero Trust model. Verify every user and device, every time.
Use micro-segmentation to limit data exposure. For large DICOM files, rely on secure, encrypted transfers with expiring download links instead of email or USB drives.
Backup, Retention & Training
- Encrypted backups ensure business continuity.
- Retention limits prevent storing data longer than needed.
- Staff training builds awareness against phishing and unsafe sharing.
How GDPR’s Data Portability Enables Patient-Controlled Image Sharing
The GDPR empowers patients with the right to data portability, allowing them to access, download, and share their medical data, shifting control from healthcare institutions to individuals.
What Data Portability Means for Patients
Under Article 20 of the GDPR, patients can:
- Receive a copy of their medical images and reports in a structured, machine-readable format (e.g., DICOM).
- Share or transfer these images directly to another healthcare professional, institution, or digital platform.
- Choose how their data moves—whether through secure download links, encrypted storage, or cloud-based access.
This right ensures patients remain in charge of their health data, fostering collaboration and continuity of care across borders.
How Providers Should Enable Secure Portability
Healthcare providers and imaging centers must design systems that support easy, secure data transfers while protecting patient privacy. Key steps include:
- Offering secure export tools for patients to download or transmit their images safely.
- Using encryption and access controls during every transfer.
- Recording each action in audit logs for accountability.
- Ensuring compliance through Data Processing Agreements (DPAs) with third-party platforms.
These safeguards ensure that convenience never compromises compliance.
Cross-Border Collaboration Made Safer
GDPR portability rights allow patients to share scans with specialists globally for second opinions. Providers must use proper transfer mechanisms.
- Standard Contractual Clauses (SCCs) or Adequacy Decisions for non-EU transfers.
- Explicit patient consent for each international share.
This approach balances accessibility with privacy, ensuring medical collaboration doesn’t cross compliance boundaries.
By combining portability with security, Medicai gives patients control without risk, making it easy to collaborate globally while keeping data safe.
Conclusion
As healthcare becomes increasingly digital and cross-border, compliance with HIPAA and GDPR ensures that privacy remains intact while collaboration thrives.
By combining encryption, audit trails, and patient control, platforms like Medicai make sharing scans for second opinions effortless and compliant.

